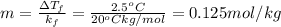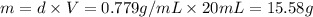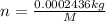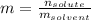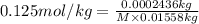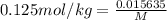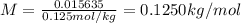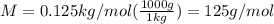Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:50
What type of reaction is illustrated? 2c12o5 = 2cl2 + 502
Answers: 2

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3
You know the right answer?
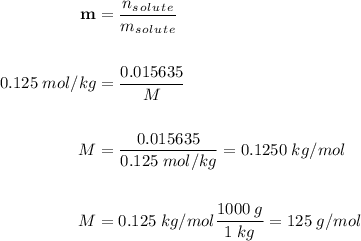
A0.2436-g sample of an unknown substance was dissolved in 20.0 ml of cyclohexane. the density of cyc...
Questions

History, 15.01.2021 22:30


Mathematics, 15.01.2021 22:30

Mathematics, 15.01.2021 22:30



History, 15.01.2021 22:30

Mathematics, 15.01.2021 22:30


Chemistry, 15.01.2021 22:30


Mathematics, 15.01.2021 22:30

Mathematics, 15.01.2021 22:30


English, 15.01.2021 22:30


English, 15.01.2021 22:30


English, 15.01.2021 22:30

 .
.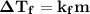
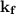 is equals to freezing point depression constant, which is equal to 20.0 ml.
is equals to freezing point depression constant, which is equal to 20.0 ml.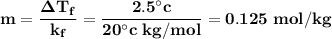
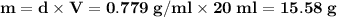
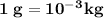
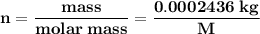
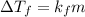
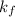 is freezing point depression constant, for cyclohexane it is equal to 20°C kg/mol.
is freezing point depression constant, for cyclohexane it is equal to 20°C kg/mol.