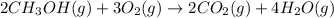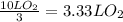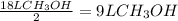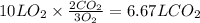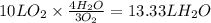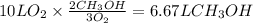Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1


Chemistry, 22.06.2019 21:00
Kp is the equilibrium constant for dissociation of the propionic acid dimer. what is the sign of the slope for a plot of the natural logarithm of kp vs. inverse temperature for this reaction?
Answers: 1
You know the right answer?
Consider the combustion of methanol at some high temperature in a constant-pressure reaction chamber...
Questions

History, 05.05.2020 03:18

Mathematics, 05.05.2020 03:18


Spanish, 05.05.2020 03:18

Mathematics, 05.05.2020 03:18


Mathematics, 05.05.2020 03:18

Mathematics, 05.05.2020 03:18

Advanced Placement (AP), 05.05.2020 03:18



Mathematics, 05.05.2020 03:18


Mathematics, 05.05.2020 03:18

English, 05.05.2020 03:18

Mathematics, 05.05.2020 03:18

Spanish, 05.05.2020 03:18

Mathematics, 05.05.2020 03:18


Social Studies, 05.05.2020 03:18