Chemistry, 10.07.2019 03:30 hallmansean04
For each of the acid–base reactions, calculate the mass (in grams) of each acid necessary to completely react with and neutralize 4.85 g of the base. a. hcl(aq) + naoh(aq) s h2o(l) + nacl(aq) b. 2 hno3(aq) + ca(oh)2(aq) s 2 h2o(l) + ca(no3)2(aq) c. h2so4(aq) + 2 koh(aq) s 2 h2o(l) + k2so4(aq)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1


Chemistry, 23.06.2019 06:00
What does it mean for something to be dissolved in watera- it is submerged in water moleculesb-it is stirred in the water moleculesc- it is surrounded by water molecules d-it has water molecules added to it
Answers: 2
You know the right answer?
For each of the acid–base reactions, calculate the mass (in grams) of each acid necessary to complet...
Questions

Mathematics, 10.10.2020 22:01



Geography, 10.10.2020 22:01


Social Studies, 10.10.2020 22:01

Mathematics, 10.10.2020 22:01


Mathematics, 10.10.2020 22:01


Mathematics, 10.10.2020 22:01




Spanish, 10.10.2020 22:01



Mathematics, 10.10.2020 22:01

Mathematics, 10.10.2020 22:01

Mathematics, 10.10.2020 22:01

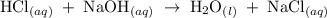
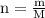
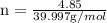
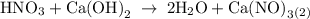
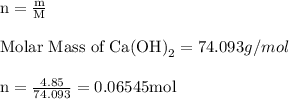
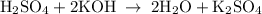

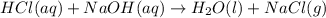
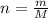
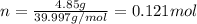
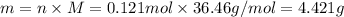
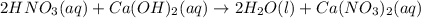
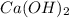 completely reacts with 2 mol of
completely reacts with 2 mol of 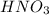 . The mass of
. The mass of 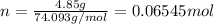
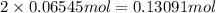 of
of 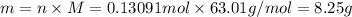
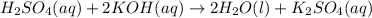
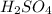 . The mass of KOH is given 4.85 g, convert this into number of moles as follows:
. The mass of KOH is given 4.85 g, convert this into number of moles as follows: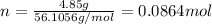
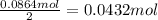 of
of