
Chemistry, 10.07.2019 03:30 jadeandryna0609
The vapor pressure of benzene, c6h6, is 100.0 torr at 26.1 °c. assuming raoult's law is obeyed, how many moles of a nonvolatile solute must be added to 100.0 ml of benzene to decrease its vapor pressure by 10.0% at 26.1 °c? the density of benzene is 0.8765 g> cm3.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 05:00
As you watch a surfer ride a wave towards the shoreline, what is the shoreline? a) displacement reference b) reference point c) coordinate plane d) cartesian boundary
Answers: 1
You know the right answer?
The vapor pressure of benzene, c6h6, is 100.0 torr at 26.1 °c. assuming raoult's law is obeyed, how...
Questions



History, 05.06.2020 00:58


Mathematics, 05.06.2020 00:58



History, 05.06.2020 00:58

Mathematics, 05.06.2020 00:58



Physics, 05.06.2020 00:58


Mathematics, 05.06.2020 00:58

Mathematics, 05.06.2020 00:58

Physics, 05.06.2020 00:58

Mathematics, 05.06.2020 00:58


Mathematics, 05.06.2020 00:58

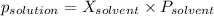 -(1)
-(1)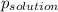 is observed vapor pressure of the solution,
is observed vapor pressure of the solution, 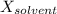 is mole fraction of solvent, and
is mole fraction of solvent, and 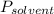 is vapor pressure of the pure solvent.
is vapor pressure of the pure solvent.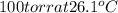 (given)
(given)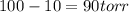
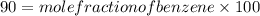
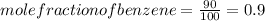
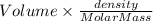
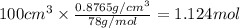
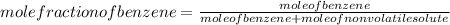
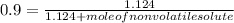
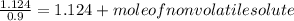
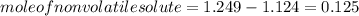
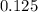 .
.

