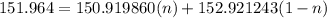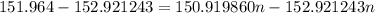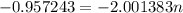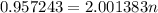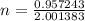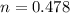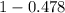Chemistry, 10.07.2019 03:30 dependentclause5828
The atomic masses of 151eu and 153eu are 150.919860 and 152.921243 amu, respectively. the average atomic mass of europium is 151.964 amu. calculate the natural abundances of these two isotopes.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Assume that the variables x and y are inversely related. if k = 18, what is the value of y for each of the following points? be sure and record your data to be used in the following problem.
Answers: 2

Chemistry, 21.06.2019 17:40
What are the resulting coefficients when you balance the chemical equation for the combustion of ethane, c2h6? in this reaction, ethane is burned in the presence of oxygen (o2) to form carbon dioxide (co2) and water (h2o). (g)+(g)→(g)+(g)
Answers: 1

Chemistry, 21.06.2019 22:30
This large tectonic plate is bounded on three sides by whats know as the ring of fire. what is the name of this tectonic plate? a) pacific plate b) eurasian plate c) north american plate d) indo- australian plate plz it's science but there's no option for science so i picked chemistry
Answers: 2

Chemistry, 22.06.2019 06:00
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1
You know the right answer?
The atomic masses of 151eu and 153eu are 150.919860 and 152.921243 amu, respectively. the average at...
Questions


Biology, 18.02.2021 01:50

Mathematics, 18.02.2021 01:50

History, 18.02.2021 01:50

History, 18.02.2021 01:50

World Languages, 18.02.2021 01:50

Chemistry, 18.02.2021 01:50


Mathematics, 18.02.2021 01:50

English, 18.02.2021 01:50

Biology, 18.02.2021 01:50

Mathematics, 18.02.2021 01:50



Biology, 18.02.2021 01:50

Mathematics, 18.02.2021 01:50

Social Studies, 18.02.2021 01:50

Physics, 18.02.2021 01:50

Mathematics, 18.02.2021 01:50

History, 18.02.2021 01:50

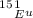 is 0.478 or 47.8% and
is 0.478 or 47.8% and 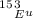 is 0.522 or 52.2% .
is 0.522 or 52.2% .