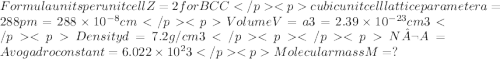Chemistry, 10.07.2019 02:00 homework2358
An element has a bcc structure with a cell edge of 288pm. the density of the element is 7.2g/cm3.how many atoms are present in 208g of the element?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Figure 10-1 study figure 10-1. the strong nuclear force felt by a single proton in a large nucleus
Answers: 3

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
You know the right answer?
An element has a bcc structure with a cell edge of 288pm. the density of the element is 7.2g/cm3.how...
Questions




Mathematics, 22.02.2020 05:45




Computers and Technology, 22.02.2020 05:45


Mathematics, 22.02.2020 05:46



Computers and Technology, 22.02.2020 05:46


Mathematics, 22.02.2020 05:46

History, 22.02.2020 05:46


History, 22.02.2020 05:46