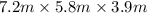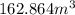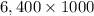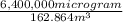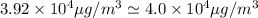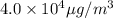Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3


Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
Nail polish remover containing acetone was spilled in a room 7.2 m × 5.8 m × 3.9 m. measurements ind...
Questions


History, 02.11.2020 17:50

Mathematics, 02.11.2020 17:50

History, 02.11.2020 17:50


Biology, 02.11.2020 17:50

Mathematics, 02.11.2020 17:50

Mathematics, 02.11.2020 17:50


Mathematics, 02.11.2020 17:50



Mathematics, 02.11.2020 17:50


Social Studies, 02.11.2020 17:50



Mathematics, 02.11.2020 17:50

Mathematics, 02.11.2020 17:50

Mathematics, 02.11.2020 17:50