
Chemistry, 10.07.2019 00:30 chrisraptorofficial
Which statement about reversible reactions is correct? at equilibrium, the forward and reverse reactions stop at the appropriate concentrations. at equilibrium, the forward and reverse reactions continue indefinitely. at equilibrium the rate of reaction of products divided by the rate of reaction of reactants equal the equilibrium constant, k.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 18:30
You open a can of soda at room temperature and hear a hiss. which of the following factors has changed inside the container? a.) atmospheric pressure b.) temperature of gas c.) type of gas d.) amount of gas
Answers: 1

You know the right answer?
Which statement about reversible reactions is correct? at equilibrium, the forward and reverse reac...
Questions

Mathematics, 17.11.2020 09:10



Chemistry, 17.11.2020 09:10




English, 17.11.2020 09:10






Mathematics, 17.11.2020 09:10

Spanish, 17.11.2020 09:10

Biology, 17.11.2020 09:10


Mathematics, 17.11.2020 09:10

Mathematics, 17.11.2020 09:10

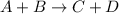
![k = \frac{[C][D]}{[A][B]}](/tpl/images/0071/3436/05b05.png)
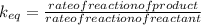
![\frac{\frac{d[C][D]}{dt}}{\frac{d[A][B]}{dt}}](/tpl/images/0071/3436/6ae5f.png)


