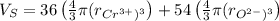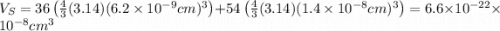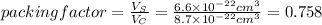Chemistry, 09.07.2019 22:00 nancye2008
The unit cell for cr2o3 has hexagonal symmetry with lattice parameters a = 0.4961 nm and c = 1.360 nm. if the density of this material is 5.22 g/cm3, calculate its atomic packing factor. the atomic weights of cr

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1

You know the right answer?
The unit cell for cr2o3 has hexagonal symmetry with lattice parameters a = 0.4961 nm and c = 1.360 n...
Questions

Health, 19.09.2019 06:01



History, 19.09.2019 06:01

Mathematics, 19.09.2019 06:01

Mathematics, 19.09.2019 06:01

Mathematics, 19.09.2019 06:01



Mathematics, 19.09.2019 06:01

Chemistry, 19.09.2019 06:01


Chemistry, 19.09.2019 06:01






Mathematics, 19.09.2019 06:01

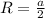
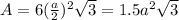
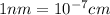
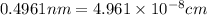
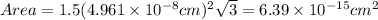
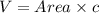
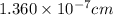
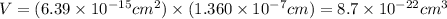
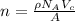
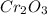 is 151.99 g/mol.
is 151.99 g/mol.
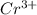 and
and 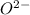 is 62 pm and 140 pm respectively.
is 62 pm and 140 pm respectively. 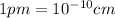
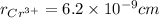
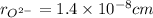
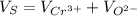
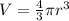 ,
,