Chemistry, 09.07.2019 21:30 brianna4455
Suppose you believe your unknown compound is naoh, and you prepare 20 ml of a 0.1 mol/l solution of it. the stockroom provides a 0.2 mol/l hcl solution as the titrant. what volume of hcl (in ml) is needed to reach the equivalence (stoichiometric) point if your unknown compound is naoh? naoh(aq) + hcl(aq) --> nacl(aq) + h2o(l)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 08:40
What is the value of keq for the reaction expressed in scientific notation?
Answers: 1

You know the right answer?
Suppose you believe your unknown compound is naoh, and you prepare 20 ml of a 0.1 mol/l solution of...
Questions


Mathematics, 03.04.2021 04:10

Health, 03.04.2021 04:10



Social Studies, 03.04.2021 04:10

Mathematics, 03.04.2021 04:10


History, 03.04.2021 04:10



Chemistry, 03.04.2021 04:10

Mathematics, 03.04.2021 04:10






History, 03.04.2021 04:10

Mathematics, 03.04.2021 04:10

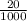 × 0.1
× 0.1