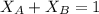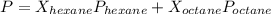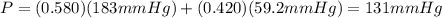Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Naoki's bicycle has a mass of 10 kg. if naoki sits on her bicycle and starts pedaling with a force of 168 n, causing an acceleration of 2.8 m/s2, what is naoki's mass?
Answers: 1

Chemistry, 22.06.2019 05:00
Which position represents spring in the southern hemisphere? a) b) c) d)
Answers: 2

Chemistry, 22.06.2019 18:00
To apply in a gold the individual gold atoms are united to each other by means of a metallic bond. how would you use the gold block to determine the atomic radius of a gold atom?
Answers: 3

Chemistry, 22.06.2019 22:20
Asuspension of yeast cells is being grown under anaerobic conditions such that glucose is degraded to ethanol and carbon dioxide. if one wishes to follow this process by monitoring the release of 14co2, at which positions in the glucose molecule would the 14c label need to be incorporated?
Answers: 2
You know the right answer?
At a given temperature the vapor pressures of hexane and octane are 183 mmhg and 59.2 mmhg, respecti...
Questions

English, 29.05.2020 00:02




Chemistry, 29.05.2020 00:02






Advanced Placement (AP), 29.05.2020 00:02


History, 29.05.2020 00:02






Chemistry, 29.05.2020 00:02

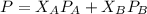
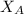 is mole fraction of A,
is mole fraction of A, 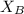 is mole fraction of B,
is mole fraction of B, 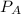 is partial pressure of A and
is partial pressure of A and  is partial pressure of B.
is partial pressure of B.