
Chemistry, 09.07.2019 21:30 BrainlyAvenger
For the reaction cyclopropane(g) → propene(g) at 500◦c, a plot of ln[cyclopropane] vs t gives a straight line with a slope of −0.00067 s−1 . what is the order of this reaction and what is the rate constant?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 20:00
Suppose that some of the compound spilled out of the crucible after it was heated. would that cause the percent by mass of water in the compound determined by the experiment to be too low, too high, or unchanged? briefly explain your answer.
Answers: 1
You know the right answer?
For the reaction cyclopropane(g) → propene(g) at 500◦c, a plot of ln[cyclopropane] vs t gives a stra...
Questions


Mathematics, 25.12.2019 01:31



Mathematics, 25.12.2019 01:31


Mathematics, 25.12.2019 01:31

History, 25.12.2019 01:31




Biology, 25.12.2019 01:31




Mathematics, 25.12.2019 01:31


History, 25.12.2019 01:31


Biology, 25.12.2019 01:31


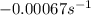 .
.![[A]=[A_{0}]e^{-kt}](/tpl/images/0070/8436/40928.png)
![[A]](/tpl/images/0070/8436/6aa06.png) is concentration of reactant at time t and
is concentration of reactant at time t and ![[A_{0}]](/tpl/images/0070/8436/747e3.png) is initial concentration of reactant.
is initial concentration of reactant.![ln[A]=ln[A_{0}]-kt](/tpl/images/0070/8436/aa7f1.png)
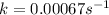 .
.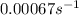 .
.![For the reaction cyclopropane(g) → propene(g) at 500◦c, a plot of ln[cyclopropane] vs t gives a stra](/tpl/images/0070/8436/bd450.jpg)


