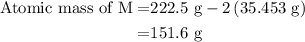Chemistry, 09.07.2019 21:00 balwinderdev
A0.999-g sample of a metal chloride, mcl2, is dissolved in water and treated with excess aqueous silver nitrate. the silver chloride that formed weighed 1.286 g. calculate the atomic mass of m.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 12:00
Which statement best explains the relationship between an area is geography and the temperature of its surface water
Answers: 1

Chemistry, 23.06.2019 06:40
A250 g sample of water with an initial temperatureof 98.8 closes 6500 joules of heat. what is the finaltemperature of the water?
Answers: 1
You know the right answer?
A0.999-g sample of a metal chloride, mcl2, is dissolved in water and treated with excess aqueous sil...
Questions

English, 28.07.2019 09:30

Biology, 28.07.2019 09:30


Biology, 28.07.2019 09:30

Business, 28.07.2019 09:30

Biology, 28.07.2019 09:30







History, 28.07.2019 09:30

Biology, 28.07.2019 09:30

English, 28.07.2019 09:30



History, 28.07.2019 09:30


Health, 28.07.2019 09:30

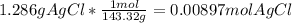

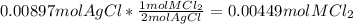
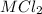 =0.999 g
=0.999 g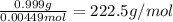
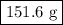 .
.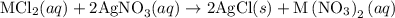
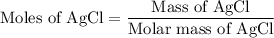 ...... (1)
...... (1)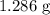 for mass of AgCl and
for mass of AgCl and 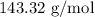 for molar mass of AgCl in equation (1).
for molar mass of AgCl in equation (1).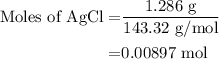
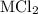 reacts with two moles of
reacts with two moles of 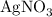 to form two moles of AgCl and one mole of
to form two moles of AgCl and one mole of 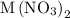 , So stoichiometric ratio between
, So stoichiometric ratio between 
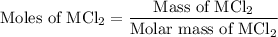 ...... (2)
...... (2)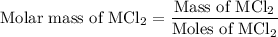 ...... (3)
...... (3)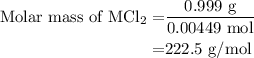
![\text{Molar mass of MCl}_2=\left[1\left(\text{Atomic mass of M}\right)+\\\\2\left(\text{Atomic mass of Cl}\right)\right]}](/tpl/images/0070/7913/9c07b.png) ....... (4)
....... (4)![\text{Atomic mass of M}=\left[\text{Molar mass of MCl}_2-2\left(\text{Atomic mass of Cl}\right)\right]](/tpl/images/0070/7913/5c489.png) ...... (5)
...... (5)