
Amixture of babr2 and inert material is analyzed to determine the ba content. first the mixture is dissolved in water. then all of the bromide ion is precipitated as agbr by the addition of an excess of silver nitrate. babr2(> 2agbr(s)+ba(no3)2(aq). in one experiment a 0.7207 g sample of the mixture resulted in 0.6226 g of agbr. determine the percent (by mass) of ba in the mixture

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Is this a scientific model? use complete sentences to explain why or why not. a graphic organizer showing the water cycle
Answers: 3

Chemistry, 21.06.2019 21:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3
You know the right answer?
Amixture of babr2 and inert material is analyzed to determine the ba content. first the mixture is d...
Questions


English, 10.03.2021 14:00

Mathematics, 10.03.2021 14:00



Mathematics, 10.03.2021 14:00


Mathematics, 10.03.2021 14:00


Mathematics, 10.03.2021 14:00


Mathematics, 10.03.2021 14:00

Mathematics, 10.03.2021 14:00




Mathematics, 10.03.2021 14:00

History, 10.03.2021 14:00

Business, 10.03.2021 14:00

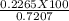 =31.42 %
.
=31.42 %
.

