
Chemistry, 09.07.2019 17:30 megansanders215
Consider the reaction below: a (aq) ↔ b (aq) kc = 2.36 if the reaction is started by placing 0.134 mol of a into 250.0 ml of solution, what will the concentration of a be at equilibrium?

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:00
What is the empirical formula of a compound that is 50.7% antimony and 49.3% selenium ?
Answers: 2

Chemistry, 23.06.2019 00:30
Quickly what are the following of organisms that existed over a wide area but only for a limited time period called a.soft fossils b.mold fossils c.index fossils d.trace fossils
Answers: 1

Chemistry, 23.06.2019 02:00
Why does ammonia, nh3, behave as a base when it reacts with an acid? z
Answers: 2

Chemistry, 23.06.2019 09:00
Describe the process that was used in this lab to create magnesium oxide, specifically identifying the type of chemical reaction. explain why the product had a higher mass than the reactant, and how this relates to conservation of matter.
Answers: 2
You know the right answer?
Consider the reaction below: a (aq) ↔ b (aq) kc = 2.36 if the reaction is started by placing 0.134...
Questions

English, 28.01.2020 16:46


English, 28.01.2020 16:46




Biology, 28.01.2020 16:46



History, 28.01.2020 16:46

History, 28.01.2020 16:46

Social Studies, 28.01.2020 16:46


Physics, 28.01.2020 16:46

Mathematics, 28.01.2020 16:46





Biology, 28.01.2020 16:46

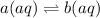
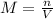
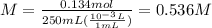
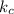 for the equilibrium reaction is:
for the equilibrium reaction is:![k_{c}=\frac{[b]}{[a]}](/tpl/images/0070/2226/ea1a2.png)
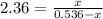
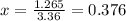
![[a]=0.536-x=0.536-0.376=0.16 M](/tpl/images/0070/2226/22b1e.png)


