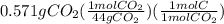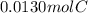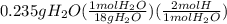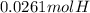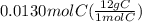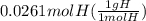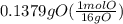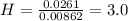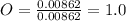Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:30
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 09:00
George is a dalmatian puppy. describe what happens to light that allows you to see george’s black and white coat.
Answers: 1

You know the right answer?
A0.320 g sample of a carboxylic acid is burned in oxygen, producing 0.571 g of co2 and 0.235 g of h2...
Questions

History, 31.08.2019 01:30

History, 31.08.2019 01:30

Mathematics, 31.08.2019 01:30

Mathematics, 31.08.2019 01:30

Mathematics, 31.08.2019 01:30

Mathematics, 31.08.2019 01:30

Mathematics, 31.08.2019 01:30

History, 31.08.2019 01:30

Mathematics, 31.08.2019 01:30


English, 31.08.2019 01:30


History, 31.08.2019 01:30

Mathematics, 31.08.2019 01:30

Biology, 31.08.2019 01:30

Mathematics, 31.08.2019 01:30

Arts, 31.08.2019 01:30

World Languages, 31.08.2019 01:30

Computers and Technology, 31.08.2019 01:30

Chemistry, 31.08.2019 01:30

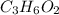 .
.