
Chemistry, 09.07.2019 17:00 qudoniselmore0
In a lithium ion battery that is discharging to power a device, for every li+ that inserts into the lithium cobalt oxide electrode, a co4+ ion must be reduced to a co3+ ion in order to balance the charge. using the crc handbook of chemistry and physics or other standard reference, find the ionic radii of li+, co3+, and co4+. order these ions from largest to smallest.

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 17:30
What is the formula for the molecular compound nitrogen monoxide
Answers: 1

Chemistry, 22.06.2019 11:30
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 22.06.2019 14:30
100 grams of molten lead (600°c) is used to make musket balls. if the lead shot is allowed to cool to room temperature (21°c), what is the change in entropy (in j/k) of the lead? (for the specific heat of molten and solid lead use 1.29 j/g⋅°c; the latent heat of fusion and the melting point of lead are 2.45 × 104 j/kg and 327°c, respectively.)
Answers: 1
You know the right answer?
In a lithium ion battery that is discharging to power a device, for every li+ that inserts into the...
Questions

Mathematics, 09.01.2022 03:20

Mathematics, 09.01.2022 03:20


Social Studies, 09.01.2022 03:20



Mathematics, 09.01.2022 03:20



Mathematics, 09.01.2022 03:20

Mathematics, 09.01.2022 03:20

Chemistry, 09.01.2022 03:20


Mathematics, 09.01.2022 03:20


Social Studies, 09.01.2022 03:30




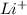 ,
, 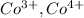 are 90 pm, 75 pm, 67 pm respectively from standard reference.
are 90 pm, 75 pm, 67 pm respectively from standard reference.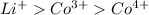 .
. ion is larger than the
ion is larger than the 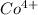 ion because as the positive charge increases on the ion then the size of the ion decreases.
ion because as the positive charge increases on the ion then the size of the ion decreases.

