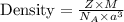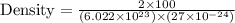Chemistry, 09.07.2019 17:00 jaallen3679
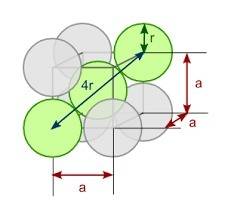
You discover a new alloy made up of cu and ni atoms. it has a bcc structure, where the ni atoms are located at the corners and the cu atoms are located in the center of each cell. the radius of the cu atoms is 0.13 nm and the radius of the ni atom is 0.15 nm. calculate the density of this structure. assume that cu has an atomic weight of 40 g/mol and ni has an atomic weight of 60 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3

Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2
You know the right answer?
You discover a new alloy made up of cu and ni atoms. it has a bcc structure, where the ni atoms are...
Questions

Mathematics, 03.12.2021 19:40

Mathematics, 03.12.2021 19:40

Business, 03.12.2021 19:40


Computers and Technology, 03.12.2021 19:40



Social Studies, 03.12.2021 19:40




Computers and Technology, 03.12.2021 19:40

Social Studies, 03.12.2021 19:40




Mathematics, 03.12.2021 19:40



SAT, 03.12.2021 19:40

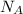 ) = 6.022 ×
) = 6.022 × 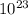
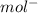
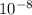 cm
cm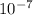 cm
cm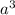 =
= 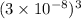 = 27 ×
= 27 × 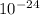
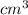
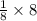 = 1atom of Ni and 1 atom of Cu
= 1atom of Ni and 1 atom of Cu