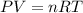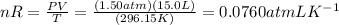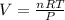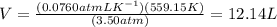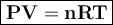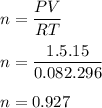Chemistry, 09.07.2019 17:00 mervesena01
Asample of cl2 gas has a volume of 15.0 l at a pressure of 1.50 atm and a temperature of 23 °c. what volume, in liters, will the gas occupy at 3.50 atm and 286 °c? assume ideal behavior.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Nickel crystallizes in the face-centered cubic (fcc) lattice. the density of the metal is 8902 kg/m3. calculate the radius of a nickel atom.
Answers: 1



Chemistry, 22.06.2019 14:30
Need ! asap will mark 10 pts using the room temperature line (orange line) and your periodic table, make lists that identify the state of matter (gas, liquid, or solid) in which each element you plotted exists at room temperature. explain your answers
Answers: 1
You know the right answer?
Asample of cl2 gas has a volume of 15.0 l at a pressure of 1.50 atm and a temperature of 23 °c. what...
Questions

Mathematics, 16.09.2020 19:01

English, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Geography, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

Mathematics, 16.09.2020 19:01

 gas= 15.0 L
gas= 15.0 L