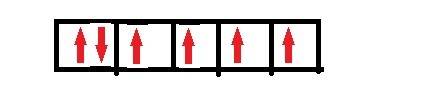
When transition metal atoms lose electrons to form ions, the electrons come first from the highest filled s atomic orbital. with this in mind , how many unpaired electrons are present in the fe2+ ion? hint: first write the outermost electron configuration of fe atom?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3


Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

You know the right answer?
When transition metal atoms lose electrons to form ions, the electrons come first from the highest f...
Questions

Biology, 01.07.2020 16:01



Mathematics, 01.07.2020 16:01

Advanced Placement (AP), 01.07.2020 16:01

Advanced Placement (AP), 01.07.2020 16:01











Mathematics, 01.07.2020 16:01