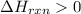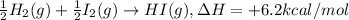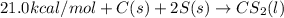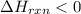Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 19:50
When the mercury level in a barometer decreases that atmospheric pressure has
Answers: 3

Chemistry, 23.06.2019 00:00
How do you determine the percent yield of a chemical reaction
Answers: 1
You know the right answer?
½h2(g) + ½i2(g) → hi(g), δh = +6.2 kcal/mole 21.0 kcal/mole + c(s) + 2s(s) → cs2(l) what type of rea...
Questions


Mathematics, 29.12.2019 03:31



Mathematics, 29.12.2019 03:31

History, 29.12.2019 03:31


English, 29.12.2019 03:31






Physics, 29.12.2019 03:31





Mathematics, 29.12.2019 03:31