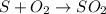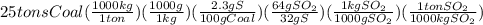Chemistry, 09.07.2019 14:00 kraigstlistt
Twenty-five tons of coal that is 2.30% sulfur by mass is burned at an electric power plant. during the combustion of the coal, the sulfur is converted into sulfur dioxide. how many tons of sulfur dioxide are produced?

Answers: 1


Another question on Chemistry


Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 21.06.2019 22:30
In order to calculate the amount of heat transferred you must know the __ and specific heat of the material, as well as the change in temperature. a. volume b. density c. mass d. enthalpy
Answers: 1

Chemistry, 21.06.2019 23:30
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i.e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3
You know the right answer?
Twenty-five tons of coal that is 2.30% sulfur by mass is burned at an electric power plant. during t...
Questions




English, 12.11.2019 02:31

Mathematics, 12.11.2019 02:31



Mathematics, 12.11.2019 02:31



History, 12.11.2019 02:31

Biology, 12.11.2019 02:31

Mathematics, 12.11.2019 02:31


Biology, 12.11.2019 02:31

English, 12.11.2019 02:31

History, 12.11.2019 02:31