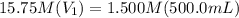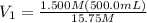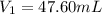Chemistry, 09.07.2019 13:30 genyjoannerubiera
Achemist needs 500.0 ml of a 1.500 m perchloric acid solution. the stockroom provides the chemist with 10.00 l of 15.75 m perchloric acid solution to prepare the required solution. calculate the volume of concentrated acid required.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2


Chemistry, 22.06.2019 22:30
Which one of the following bonds would you expect to be the most polar? a) b–h b) n–h c) p–h d) al–h e) c–h
Answers: 1

Chemistry, 23.06.2019 02:30
When the ionic compound nabr dissolves in water, br– ions are pulled into solution by the attraction between what two particles? a. the na+ and br– ions b. the na+ ion and the negative end of a water molecule c. the br– ion and the positive end of a water molecule d. the br– ion and the negative end of a water molecule
Answers: 1
You know the right answer?
Achemist needs 500.0 ml of a 1.500 m perchloric acid solution. the stockroom provides the chemist wi...
Questions

Geography, 20.11.2020 19:50


English, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50

Biology, 20.11.2020 19:50

Mathematics, 20.11.2020 19:50



Mathematics, 20.11.2020 19:50


Mathematics, 20.11.2020 19:50


Social Studies, 20.11.2020 19:50

Arts, 20.11.2020 19:50




Mathematics, 20.11.2020 19:50

Computers and Technology, 20.11.2020 19:50

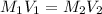
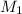 is the concentration of the concentrated solution and
is the concentration of the concentrated solution and 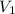 is it's volume.
is it's volume. 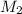 is the concentration of the diluted solution and
is the concentration of the diluted solution and 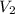 is it's volume. Let's plug in the values in the equation and solve it for
is it's volume. Let's plug in the values in the equation and solve it for