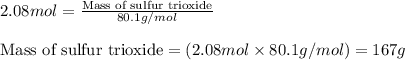Chemistry, 09.07.2019 12:30 doggosbepis
The reaction of sulfur with oxygen is shown below. 2s(s) + 3o2(g) → 2so3(g) calculate the mass of sulfur trioxide (in g) produced when 100.0 g of each reactant is present.

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1

Chemistry, 23.06.2019 06:30
Which of these describes how heat is transferred by convection* a. sunlight travels through space without the aid of fluids or solids. b. warm air rises and takes the heat with it, eventually, it cools and sinks c. air at the equator rises and sinks at the poles. d. air molecules touch the warm ground, heating them up *not conduction
Answers: 3

Chemistry, 23.06.2019 07:30
Assume that 13.5 g solid aluminum (al) react with hcl to produce solid aluminum chloride (alcl3) salt and gaseous hydrogen (h2) at standard temperature and pressure.
Answers: 1
You know the right answer?
The reaction of sulfur with oxygen is shown below. 2s(s) + 3o2(g) → 2so3(g) calculate the mass of su...
Questions



English, 20.12.2021 23:20





Advanced Placement (AP), 20.12.2021 23:20


History, 20.12.2021 23:20

Mathematics, 20.12.2021 23:20

Geography, 20.12.2021 23:20





Mathematics, 20.12.2021 23:20

Mathematics, 20.12.2021 23:20


Mathematics, 20.12.2021 23:20

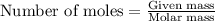 .....(1)
.....(1)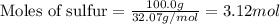
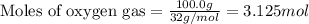
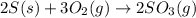
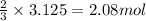 of sulfur metal
of sulfur metal