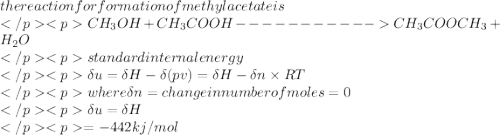Chemistry, 19.09.2019 16:50 clevelandjaniya1
Estimate the standard internal energy of formation of liquid methyl acetate (methyl ethanoate, ch3cooch3) at 298 k from its standard enthalpy of formation, which is –442 kj mol-1.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 12:30
Which element has the lowest electronegativity? calcium(ca) gallium(ga) selenium(se) bromine(br)
Answers: 1

Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2

Chemistry, 23.06.2019 06:10
2. what two items do autotrophs take from the environment to produce their food? 3. what are the two items that are released during transpiration from leaves? 4. what are the two membranes of the system? a.what are the two stages of photosynthesis? what are the two parts of photosynthesis?
Answers: 2
You know the right answer?
Estimate the standard internal energy of formation of liquid methyl acetate (methyl ethanoate, ch3co...
Questions

Mathematics, 12.04.2021 22:10






Chemistry, 12.04.2021 22:10

Mathematics, 12.04.2021 22:10

Mathematics, 12.04.2021 22:10

English, 12.04.2021 22:10

Mathematics, 12.04.2021 22:10





Mathematics, 12.04.2021 22:10




English, 12.04.2021 22:10