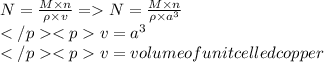To calculate avogadro's number from the measured density of copper shot, what additional information is not needed? the molar mass of copper the atomic number of copper the length of the edge of the unit cell of copper the number of copper atoms occupying one unit cell of copper

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:30
When the earth was formed and cooled, why did nickel and iron end up in the center of the earth while basalt and granite ended up in the outer layers
Answers: 3

Chemistry, 22.06.2019 06:30
The best solution for preventing harm to people and pets from severe hurricanes involves determining and warning residents about what
Answers: 1

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3
You know the right answer?
To calculate avogadro's number from the measured density of copper shot, what additional information...
Questions


Arts, 18.03.2021 02:50


Chemistry, 18.03.2021 02:50

Physics, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50

English, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50

English, 18.03.2021 02:50


Advanced Placement (AP), 18.03.2021 02:50

Health, 18.03.2021 02:50

Mathematics, 18.03.2021 02:50


Mathematics, 18.03.2021 02:50


Mathematics, 18.03.2021 02:50