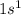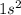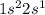Chemistry, 09.07.2019 12:00 loravillanueva87
Which set of these comparisons is incorrect? (more stable means it has a more negative energy.) a. the 1s orbital in h is more stable than the 1s orbital in he+ b. the 2s orbital in he atom is less stable than the 2s orbital in he+ c. the 2s subshell in li is more stable than the 2p subshell in li?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
If 200.0g of copper(ll) sulfate react with an excess of zinc metal, what is the theoretical yield of copper
Answers: 1

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 23.06.2019 00:30
What would be the original temperature of a gas that has a volume of 2.0 l and a pressure of 2.0 atm and an unknown temperature that the volume increased to 3.5 l in its pressure decreased to 1.0 atm if the final temperature is measured to be 11°c
Answers: 1
You know the right answer?
Which set of these comparisons is incorrect? (more stable means it has a more negative energy.) a....
Questions

Chemistry, 10.02.2021 06:50

Mathematics, 10.02.2021 06:50


Business, 10.02.2021 06:50

Mathematics, 10.02.2021 06:50


Mathematics, 10.02.2021 06:50

Mathematics, 10.02.2021 06:50

Social Studies, 10.02.2021 06:50


Mathematics, 10.02.2021 06:50






Mathematics, 10.02.2021 06:50

Mathematics, 10.02.2021 06:50

Mathematics, 10.02.2021 06:50