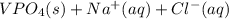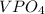Chemistry, 09.07.2019 08:00 aaronlikly
Two solutions are mixed, bringing together ions of na+, po43−, v3+, and cl−. use the solubility rules to identify the composition of the salt that precipitates out of the solution. a. vpo4 b. na3po4 c. vcl3 d. nacl

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 21:30
Athe top of a hill, an athlete on a skateboard has x joules of mechanical energy. how much mechanical energy will she have at the bottom of the hill? ignore the effects of friction.
Answers: 1

Chemistry, 23.06.2019 02:00
As light moves from one material into the next, which of the following affects how much the light waves will refract, or bend? angle at which the ray strikes the medium color of the material density of the material temperature of the light wave
Answers: 2
You know the right answer?
Two solutions are mixed, bringing together ions of na+, po43−, v3+, and cl−. use the solubility rule...
Questions

Mathematics, 17.07.2019 19:00

Physics, 17.07.2019 19:00



Mathematics, 17.07.2019 19:00


English, 17.07.2019 19:00



Mathematics, 17.07.2019 19:00




History, 17.07.2019 19:00

Health, 17.07.2019 19:00

Mathematics, 17.07.2019 19:00

Biology, 17.07.2019 19:00


Health, 17.07.2019 19:00

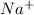 ,
,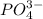 ,
,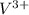 , and
, and 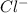 . The products formed in the reaction would be NaCl and VPO
. The products formed in the reaction would be NaCl and VPO . As per the solubiltiy rules, all chloride salts are soluble, all phosphate salts are insoluble except those with
. As per the solubiltiy rules, all chloride salts are soluble, all phosphate salts are insoluble except those with 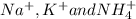 .
.