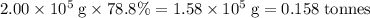Bornite (cu3fes3) is a copper ore used in the production of copper. when heated, the following reaction occurs. 2 cu3fes3(s) + 7 o2(g) 6 cu(s) + 2 feo(s) + 6 so2(g) if 3.24 metric tons of bornite is reacted with excess o2 and the process has an 78.8% yield of copper, what mass of copper is produced?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:20
2pos suppose an object in free fall is dropped from a building. its starting velocity is 0 m/s. ignoring the effects of air resistance, what is the speed (in m/s) of the object after falling 3 seconds? give your answer as a positive decimal without units. answer here
Answers: 1

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 19:20
For a research project, a student decided to test the effect of the lead(ii) ion (pb2+) on the ability of salmon eggs to hatch. this ion was obtainable from the water‐soluble salt, lead(ii) nitrate, which the student decided to make by the following reaction. pbo(s) + 2 hno3(aq) → pb(no3)2(aq) + h2o losses of product for various reasons were expected, and a yield of 86.0% was expected. in order to have 5.00 g of product at this yield, how many grams of pbo should be reacted? (assume that sufficient nitric acid, hno3, would be used.)
Answers: 1
You know the right answer?
Bornite (cu3fes3) is a copper ore used in the production of copper. when heated, the following react...
Questions

Biology, 08.10.2019 20:00

Health, 08.10.2019 20:00

Mathematics, 08.10.2019 20:00

History, 08.10.2019 20:00



English, 08.10.2019 20:00



Chemistry, 08.10.2019 20:00




Biology, 08.10.2019 20:10






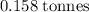
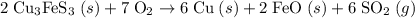
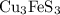 has a molar mass of
has a molar mass of 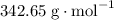 .
. 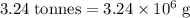 of bornite would thus contain
of bornite would thus contain 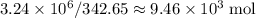 formula units of this substance. Each two formula unit of bornite combusts to produce six formula units of copper metal
formula units of this substance. Each two formula unit of bornite combusts to produce six formula units of copper metal 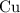 as seen in the equation;
as seen in the equation; 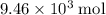 of bornite would therefore produce
of bornite would therefore produce 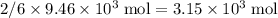 of copper, which corresponds to a theoretical yield of
of copper, which corresponds to a theoretical yield of 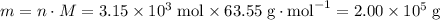 .
. 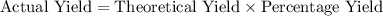 .
.