
Chemistry, 09.07.2019 04:30 ryrytkg5107
Consider the reaction cl2(g) + i2(g) < => 2 icl(g), which is endothermic as written. what would be the effect on the equilibrium position of removing cl2(g)? 1. reaction would go to the left, making more "reactants" 2. no change on the equilibrium position 3. reaction would go to the right, making more "reactants" 4. reaction would go to the left, making more "products" 5. reaction would go to the right, making more "products"

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Alarge marble is dropped in a graduated cylinder with 35ml of water in it.the water level increases to 49ml.what is the volume of the marble
Answers: 1

Chemistry, 22.06.2019 05:50
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 15:30
Each of the following reactions is allowed to come to equilibrium and then the volume is changed as indicated. predict the effect (shift right, shift left, or no effect) of the indicated volume change. drag the appropriate items to their respective bins.co(g) + h2o(g) < => co2(g) + h2(g) (volume is decreased) pcl3(g) + cl2(g) < => pcl5(g) (volume is increased) caco3(s)< => cao(s) + co2(g) (volume is increased)
Answers: 1
You know the right answer?
Consider the reaction cl2(g) + i2(g) < => 2 icl(g), which is endothermic as written. what wou...
Questions

History, 29.08.2019 03:00


Chemistry, 29.08.2019 03:00

Chemistry, 29.08.2019 03:00


Physics, 29.08.2019 03:00


Mathematics, 29.08.2019 03:00

Mathematics, 29.08.2019 03:00

Mathematics, 29.08.2019 03:00

Social Studies, 29.08.2019 03:00


Social Studies, 29.08.2019 03:00

Biology, 29.08.2019 03:00


Biology, 29.08.2019 03:00



Biology, 29.08.2019 03:00

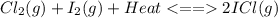
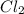 is removed from the system, the concentration of chlorine (
is removed from the system, the concentration of chlorine (

