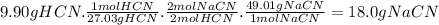Chemistry, 09.07.2019 04:30 emily41950
Consider the formation of hcn by the reaction of nacn (sodium cyanide) with an acid such as h2so4 (sulfuric acid): 2nacn(s)+h2so4(aq)âna2so4(aq)+2hcn( g) what mass of nacn gives the lethal dose in the room?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
This is a characteristic of the elements in the periodic table that shows a pattern. it may increase or decrease across or down the table.
Answers: 1

Chemistry, 22.06.2019 02:40
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
Consider the formation of hcn by the reaction of nacn (sodium cyanide) with an acid such as h2so4 (s...
Questions

Mathematics, 19.05.2021 18:00

Mathematics, 19.05.2021 18:00

Mathematics, 19.05.2021 18:00



Mathematics, 19.05.2021 18:00


Biology, 19.05.2021 18:00

Chemistry, 19.05.2021 18:00

English, 19.05.2021 18:00


Mathematics, 19.05.2021 18:00


Engineering, 19.05.2021 18:00

Mathematics, 19.05.2021 18:00

Mathematics, 19.05.2021 18:00

Business, 19.05.2021 18:00


Mathematics, 19.05.2021 18:00

Mathematics, 19.05.2021 18:00