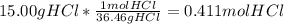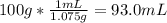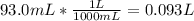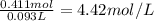Chemistry, 09.07.2019 04:00 shelby7629
The mass percentage of hydrochloric acid within a solution is 15.00%. given that the density of this solution is 1.075 g/ml, find the molarity of the solution.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:40
Astudent is given a sample of a blue copper sulfate hydrate. he weighs the sample in a dry covered porcelain crucible and got a mass of 23.875 g for the crucible, lid, and sample. the mass of the empty crucible and lid was found earlier to be 22.652 g. he then heats the crucible to expel the water of hydration, keeping the crucible at red heat for 10 minutes with the lid slightly ajar. on colling, he finds the mass of crucible, lid, and contents to be 23.403 g. the sample was changed in the process to very light clue anhydrous cuso4. if there are again 100.0 g of hydrate, how many grams of cuso4 are in it? how many moles of cuso4? (hint: molar mass of cuso4 = 159.6 g / mole. what per cent of the hydrate is cuso4? you may convert the mass of cuso4 to moles.)
Answers: 3

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

Chemistry, 23.06.2019 16:30
In chile, the deepest earthquake occurred at 61.7°w longitude at a depth of 540 km. if the rocks at the focus began subducting 10 million years ago and are now 1000 km from their original position, what is the average rate of subduction in cm/yr?
Answers: 2
You know the right answer?
The mass percentage of hydrochloric acid within a solution is 15.00%. given that the density of this...
Questions






Computers and Technology, 20.09.2019 17:10







Mathematics, 20.09.2019 17:20