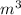Chemistry, 06.10.2019 12:00 biancaadenisee2
The equilibrium fraction of lattice sites that are vacant in silver (ag) at 500â°c is 0.5 ã 10-6. calculate the number of vacancies (per meter cubed) at 500â°c. assume a density of 10.35 g/cm3 for ag, and note that aag = 107.87 g/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:30
How many grams of phosphorus are contained in 5.09 moles of phosphorus?
Answers: 1

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3

Chemistry, 23.06.2019 04:20
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1

Chemistry, 23.06.2019 09:00
Which of the following are in a chemical family a. ca, sc, k b. cu, ag, au c. so, ge, sb
Answers: 1
You know the right answer?
The equilibrium fraction of lattice sites that are vacant in silver (ag) at 500â°c is 0.5 ã 10-6. ca...
Questions


Mathematics, 31.12.2020 09:10

Chemistry, 31.12.2020 09:10

Chemistry, 31.12.2020 09:10



English, 31.12.2020 09:10

Mathematics, 31.12.2020 09:10

Mathematics, 31.12.2020 09:10

Mathematics, 31.12.2020 09:10


Chemistry, 31.12.2020 09:10

Mathematics, 31.12.2020 09:10

Chemistry, 31.12.2020 09:10

Mathematics, 31.12.2020 09:20

Mathematics, 31.12.2020 09:20

English, 31.12.2020 09:20

Chemistry, 31.12.2020 09:20

Chemistry, 31.12.2020 09:20

Mathematics, 31.12.2020 09:20

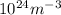
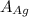 = 107.87 g/mol
= 107.87 g/mol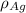 = 10.35
= 10.35 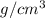 = 10.35
= 10.35 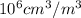 = 10.35 ×
= 10.35 × 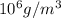
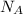 = 6.022 ×
= 6.022 × 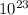 atoms/mol
atoms/mol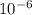
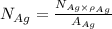
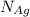 = Total number of lattice sites in Ag
= Total number of lattice sites in Ag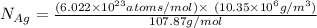
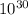 atoms/
atoms/