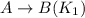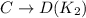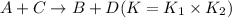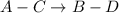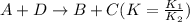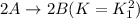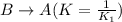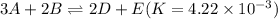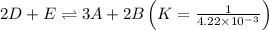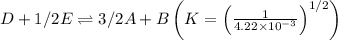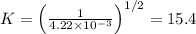Chemistry, 09.07.2019 01:00 sebasm4842
The equilibrium constant for the equilibrium, 3a+ 2b ↔ 2d + e, is 4.22 x 10-3 . what is the equilibrium constant for the equilibrium: d + (1/2)e ↔ (3/2)a + b? 2.1110-3237-2.1110-315.4

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:30
An empty fuel tank can still contain and therefore can be even more dangerous than one full of liquid fuel.
Answers: 1

Chemistry, 23.06.2019 06:20
Examine the false statement. compounds are the smallest unit of an element that occur most commonly in nature. select the rewording of the statement that is true. a: atoms are the smallest unit of an element that commonly occur in nature. b: molecules are the smallest unit of an element or compound that commonly occur in nature. c: molecules are the smallest unit of a compound that occur on the periodic table. d: compounds are the smallest unit of an element that occur on the periodic table
Answers: 1

Chemistry, 23.06.2019 07:40
What is the reduction potential of a hydrogen electrode that is still at standard pressure, but has ph = 5.65 , relative to the she?
Answers: 1

Chemistry, 23.06.2019 15:00
In two or more complete sentences describe all of the van der waals forces that exist between molecules of sulfur dioxide, so2.
Answers: 1
You know the right answer?
The equilibrium constant for the equilibrium, 3a+ 2b ↔ 2d + e, is 4.22 x 10-3 . what is the eq...
Questions


Mathematics, 20.04.2021 19:00


English, 20.04.2021 19:00

Mathematics, 20.04.2021 19:00




Mathematics, 20.04.2021 19:00





Computers and Technology, 20.04.2021 19:00


English, 20.04.2021 19:00



Mathematics, 20.04.2021 19:00

Advanced Placement (AP), 20.04.2021 19:00