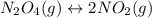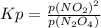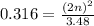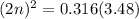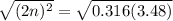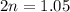Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1


Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
For the equilibrium, n2o4(g)< > 2no2(g), the equilibrium constant kp = 0.316. calculate the e...
Questions


Mathematics, 25.02.2021 21:00

Mathematics, 25.02.2021 21:00

Mathematics, 25.02.2021 21:00

Chemistry, 25.02.2021 21:00




Mathematics, 25.02.2021 21:00

Mathematics, 25.02.2021 21:00


Mathematics, 25.02.2021 21:00



Mathematics, 25.02.2021 21:00

Mathematics, 25.02.2021 21:00


Mathematics, 25.02.2021 21:00

Mathematics, 25.02.2021 21:00

History, 25.02.2021 21:00

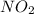 is 1.05 atm.
is 1.05 atm.