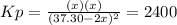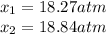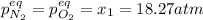Chemistry, 08.07.2019 23:30 juansebas35
for the equilibrium: 2 no (g) < > n2(g) + o2 (g), kp=2400. if initially, only no is present at a partial pressure of 37.30 atm, what will the partial pressures of n2 and o2 be at equilibrium? 1827 atm 38.08 atm 1.725 atm 36.55 atm

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 00:30
Drive down any three characteristic of modern periodic table
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3
You know the right answer?
for the equilibrium: 2 no (g) < > n2(g) + o2 (g), kp=2400. if initially, only no is pres...
Questions

Chemistry, 30.12.2019 15:31


Biology, 30.12.2019 15:31

Mathematics, 30.12.2019 15:31

Mathematics, 30.12.2019 15:31

Social Studies, 30.12.2019 15:31



Chemistry, 30.12.2019 15:31





Mathematics, 30.12.2019 15:31

Biology, 30.12.2019 15:31




Chemistry, 30.12.2019 15:31

Mathematics, 30.12.2019 15:31

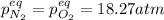
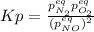
 , changes to:
, changes to: