
Chemistry, 08.07.2019 21:00 khadythiam6957
When solid phosphorus (p4) reacts with oxygen gas, diphosphorus pentoxide is formed. initially 2.87 mol of phosphorus and 3.86 mol of oxygen are combined. (assume 100% yield). a) write a balanced equation for the reaction. b) what is the limiting reactant? c) how many miles of excess reactant remain after the reaction is complete?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 11:50
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3

Chemistry, 23.06.2019 12:30
Idid a lab for chemistry where we put nails in a copper (ii) chloride solution. 1. why did the reaction stop? which reactant was used up? how do you know? 2. describe what was happening to the atoms of iron and copper during the reaction. what is this type of reaction called? 3. what would happen to the ratio of copper to iron if you had placed more nails in the beaker? if you had let the reaction go for less time? 4. what is the accepted ratio of copper atoms to iron atoms in this reaction? account for differences between your experimental value and the accepted value. write the balanced equation for the reaction.
Answers: 2

Chemistry, 23.06.2019 15:00
How much more basic is a solution with ph 8 than a solution with ph 7
Answers: 1
You know the right answer?
When solid phosphorus (p4) reacts with oxygen gas, diphosphorus pentoxide is formed. initially 2.87...
Questions

Mathematics, 28.01.2020 04:31


Computers and Technology, 28.01.2020 04:31

Social Studies, 28.01.2020 04:31













Mathematics, 28.01.2020 04:31


Mathematics, 28.01.2020 04:31

Mathematics, 28.01.2020 04:31

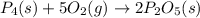
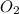 .
.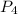 is 0.94 moles.
is 0.94 moles.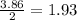 moles of
moles of 

