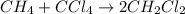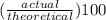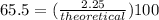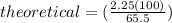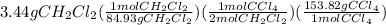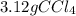Chemistry, 08.07.2019 18:00 bionicboy03120440
It is desired to produce 2.25 grams of dichloromethane (ch2cl2) by the following reaction. if the percent yield of dichloromethane (ch2cl2) is 65.5 %, how many grams of carbon tetrachloride would need to be reacted?carbon tetrachloride methane (ch4)(g) + carbon tetrachloride(g) dichloromethane (ch2cl2)(g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 08:30
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

You know the right answer?
It is desired to produce 2.25 grams of dichloromethane (ch2cl2) by the following reaction. if the pe...
Questions




Mathematics, 30.09.2019 00:20


Mathematics, 30.09.2019 00:20



Mathematics, 30.09.2019 00:20

Mathematics, 30.09.2019 00:20


English, 30.09.2019 00:20


Mathematics, 30.09.2019 00:20

English, 30.09.2019 00:20



History, 30.09.2019 00:20

Physics, 30.09.2019 00:20

Mathematics, 30.09.2019 00:20