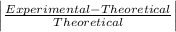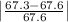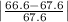Student a performed gravimetric analysis for sulfate in her unknown using the same procedures we did . results of her three trials were 68.6%, 66.2% and 67.1% sulfate. student b analyzed the same unknown his results were 66.7%, 66.6% and 66.5%. the unknown was sodium sulfate. calculate a percent e rror fo r student a and for student b using as the accepted value the theoretical value for sulfate in sodium sulfate based on molar mass . [y ou may use the internet, a textbook, the chemistry c ommunity learning center (cclc) or any other resource for an explanation/description of percent error .] which student , a or b, was more accurate? which student was more precise? explain your answers

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1


Chemistry, 23.06.2019 00:10
Find the missing probability in the table below a.0.10 b.40 c.0.80 d. 0.20
Answers: 2
You know the right answer?
Student a performed gravimetric analysis for sulfate in her unknown using the same procedures we did...
Questions


Mathematics, 02.02.2020 23:04


Social Studies, 02.02.2020 23:04



Mathematics, 02.02.2020 23:04


Chemistry, 02.02.2020 23:04



Mathematics, 02.02.2020 23:05

History, 02.02.2020 23:05

Mathematics, 02.02.2020 23:05

Mathematics, 02.02.2020 23:05



History, 02.02.2020 23:05