
Chemistry, 04.02.2020 21:48 ballislifeqw3089
An atom's lewis dot structure has three dots. which of the following elements could it be, and why? aluminum, because it is in group 13 and has three valence electrons. lithium, because it is a group 1 element with three total electrons. magnesium, because it is in period 3 and has three valence electrons. potassium, because it is an alkali metal and has three inner shell electrons.
question 2(multiple choice worth 4 points) (03.01 lc) an atom has the following electron configuration. 1s22s22p63s23p3 how many valence electrons does this atom have? 3 5 8 15 question 3 (matching worth 4 points) (03.01 lc) match each element to the number of electrons in its valence shell. match term definition chlorine (cl) a) eight neon (ne) b) five phosphorus (p) c) seven sulfur (s) d) six question 4 (true/false worth 3 points) (03.01 lc) an atom's valence electrons are located in the atom's outermost energy level. true false question 5 (essay worth 5 points) (03.01 mc) the electron configuration of an element is 1s22s22p63s1. describe what most likely happens when an atom of this element comes near an atom having seven valence electrons.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3
You know the right answer?
An atom's lewis dot structure has three dots. which of the following elements could it be, and why?...
Questions

Chemistry, 12.10.2019 19:00

Mathematics, 12.10.2019 19:00

Spanish, 12.10.2019 19:00




Computers and Technology, 12.10.2019 19:00


Mathematics, 12.10.2019 19:00


Mathematics, 12.10.2019 19:00

History, 12.10.2019 19:00


Mathematics, 12.10.2019 19:00


Mathematics, 12.10.2019 19:00

Mathematics, 12.10.2019 19:00

Biology, 12.10.2019 19:00

Business, 12.10.2019 19:00

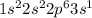 , has an unpaired electron in the 3s orbital which makes it unstable, while the element with 7 valence electrons needs 1 more electron to achieve an octet. Henceforth, the
, has an unpaired electron in the 3s orbital which makes it unstable, while the element with 7 valence electrons needs 1 more electron to achieve an octet. Henceforth, the 


