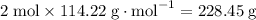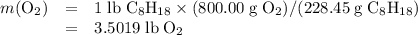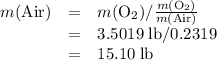Chemistry, 08.07.2019 11:00 swaggg8300
Air is 23.19% o2 and 75.46% n2 by weight) would be needed to burn a pound of gasoline by a reaction whereby c8h18 reacts with o2 to form co2 and h2o?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3


Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2
You know the right answer?
Air is 23.19% o2 and 75.46% n2 by weight) would be needed to burn a pound of gasoline by a reaction...
Questions


Computers and Technology, 21.01.2020 05:31

Social Studies, 21.01.2020 05:31


Business, 21.01.2020 05:31

Chemistry, 21.01.2020 05:31










Computers and Technology, 21.01.2020 05:31


Computers and Technology, 21.01.2020 05:31


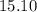 pounds.
pounds.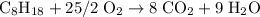
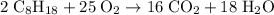
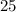 molecules of oxygen to completely react with
molecules of oxygen to completely react with 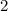 molecules of octane. Oxygen and octane thus react at a ratio of
molecules of octane. Oxygen and octane thus react at a ratio of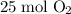 to
to 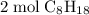
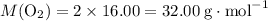
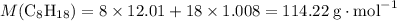
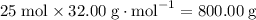 to
to