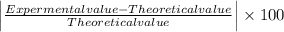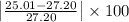Milhouse massed a copper cylinder multiple times. his data is listed below. the "correct" mass of the cylinder had been previously determined to be 27.20 grams. desrcibe the accuracy and precision of the milhouses measurements. weighing 1 - 25.01weighing 2 - 25.22weighing 3 - 25.23weighing 4 - 25.19(part 2) refer to the data above. what was the percent error on milhouses first weighing? percent error = l experimental value - accepted value l / accepted value x 100%

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:30
Apure solvent has a vapor pressure the vapor pressure of a solution. a. equal to b. lower than c. higher than
Answers: 1

Chemistry, 21.06.2019 22:00
Which describes interactions between substances and stomata during photosynthesis? check all that apply. oxygen enters stomata. oxygen is released through stomata. carbon dioxide enters stomata. carbon dioxide is released through stomata. hydrogen enters stomata. hydrogen is released through stomata.
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 15:10
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion.what is the frequency f of oscillation?
Answers: 2
You know the right answer?
Milhouse massed a copper cylinder multiple times. his data is listed below. the "correct" mass of th...
Questions


Spanish, 01.05.2021 16:40


Mathematics, 01.05.2021 16:40








Mathematics, 01.05.2021 16:40


Business, 01.05.2021 16:40




English, 01.05.2021 16:40