Chemistry, 08.07.2019 08:00 supergraciepie
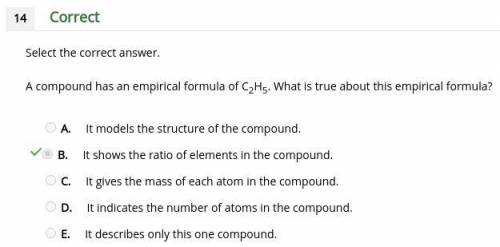
Acompound has an empirical formula of c2h5. what is true about this empirical formula? a. it models the structure of the compound. b. it shows the ratio of elements in the compound. c. it gives the mass of each atom in the compound. d. it indicates the number of atoms in the compound. e. it describes only this one compound.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1



Chemistry, 22.06.2019 06:00
One of the few xenon compounds that form is cesium xenon heptafluoride (csxef7). how many moles of csxef7 can be produced from the reaction of 13.0 mol cesium fluoride with 12.5 mol xenon hexafluoride? csf(s) + xef6(s) csxef7(s)
Answers: 1
You know the right answer?
Acompound has an empirical formula of c2h5. what is true about this empirical formula? a. it models...
Questions




Biology, 02.08.2019 19:40

History, 02.08.2019 19:40


Biology, 02.08.2019 19:40

Chemistry, 02.08.2019 19:40

Mathematics, 02.08.2019 19:40




English, 02.08.2019 19:40

Health, 02.08.2019 19:40

Mathematics, 02.08.2019 19:40

Social Studies, 02.08.2019 19:40

Mathematics, 02.08.2019 19:40

History, 02.08.2019 19:40