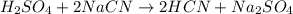Chemistry, 25.11.2019 21:31 melissarodrigue7
Question 1(multiple choice worth 4 points)
(05.04 mc)
what mass of co is needed to react completely with 55.0 g of fe2o3 in the reaction: fe2o3(s) + co(g) → fe(s) + co2(g)?
4.82 g co
9.64 g co
14.5 g co
28.9 g co
question 2(multiple choice worth 4 points)
(05.04 lc)
which of the following is a valid mole ratio from the balanced equation 2c3h6 + 9o2 → 6co2 + 6h2o?
one mole of c three h six over two moles of c o two
six moles of h two o over nine moles of o two
two moles of c three h six over six moles of o two
three moles of h two o over 2 moles of c o two
question 3(multiple choice worth 4 points)
(05.04 mc)
read the following chemical equation.
fe + o2 → fe2o3
which of the following fractions can be used for the mole ratio to determine the mass of fe from a known mass of fe2o3?
four over two
three over two
two over three
two over four
question 4(multiple choice worth 4 points)
(05.04 mc)
in an experiment, zinc chlorate decomposed according to the following chemical equation.
zn(clo3)2 → zncl2 + o2
(molar mass of zn(clo3)2 = 232.29 g/mol; zncl2 = 136.286 g/mol; o2 = 31.998 g/mol)
if the mass of zinc chlorate was 150 grams, which of the following calculations can be used to determine the mass of oxygen gas formed?
(150 × 1 × 232.29) ÷ (31.998 × 3) grams
(150 × 3 × 232.29) ÷ (31.998 × 1) grams
(150 × 1 × 31.998) ÷ (232.29 × 3) grams
(150 × 3 × 31.998) ÷ (232.29 × 1) grams
question 5(multiple choice worth 4 points)
(05.04 lc)
how many moles of sodium cyanide (nacn) would be needed to produce 4.2 moles of sodium sulfate (na2so4)?
h2so4 + 2nacn → 2hcn + na2so4
2.1 mol nacn
4.2 mol nacn
8.4 mol nacn
12.0 mol nacn

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
You encounter a solution that is acidic and you decide to test it by adding a small amount of a strong acid. the ph lowers slightly but is approximately unchanged, and still remains acidic. what can you say about the solution? a. it is a buffer solution. b. it is not a buffer solution it is a strong acid solution. d. the solution has been neutralized. e. the solution has excess acid present
Answers: 1

Chemistry, 22.06.2019 09:00
Acrystal that absorvd water from air is (blank)a. aqueousb. homogenousc. hygroscopicd. efflorescent
Answers: 1

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 23.06.2019 06:00
What does it mean for something to be dissolved in watera- it is submerged in water moleculesb-it is stirred in the water moleculesc- it is surrounded by water molecules d-it has water molecules added to it
Answers: 2
You know the right answer?
Question 1(multiple choice worth 4 points)
(05.04 mc)
what mass of co is needed t...
(05.04 mc)
what mass of co is needed t...
Questions



Mathematics, 02.09.2021 20:50


Biology, 02.09.2021 20:50

Health, 02.09.2021 20:50

Mathematics, 02.09.2021 20:50





Mathematics, 02.09.2021 20:50

Mathematics, 02.09.2021 20:50



Mathematics, 02.09.2021 20:50


Mathematics, 02.09.2021 20:50

Mathematics, 02.09.2021 20:50

 over Nine moles of
over Nine moles of 
 .
.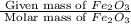 =
= 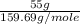 = 0.344 moles
= 0.344 moles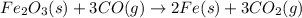
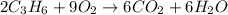
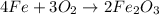
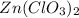 = 150 g
= 150 g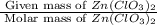 =
= 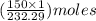
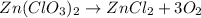
![[(\frac{150\times 1}{232.29})\times 3] moles](/tpl/images/0390/3270/cd451.png) of
of ![[(\frac{150\times 1}{232.29})\times 3] \times 31.998 grams](/tpl/images/0390/3270/9e58a.png)
 = 4.2 moles
= 4.2 moles