
Chemistry, 08.07.2019 06:00 sotoamerica0814
In the atmosphere, under the effect of ultraviolet rays, oxygen molecules combine to form ozone molecules, and ozone molecules also photodissociate to form oxygen molecules. which of the following would be observed if this reaction is in dynamic equilibrium? the concentration of oxygen will increase. the concentration of ozone will decrease. the rates of the forward and reverse reactions will increase. the rates of the forward and reverse reactions will remain the same.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2

Chemistry, 22.06.2019 08:30
How would the number of moles (n) of o2 change if the atmospheric pressure doubled but all other variables stayed the same
Answers: 2

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3
You know the right answer?
In the atmosphere, under the effect of ultraviolet rays, oxygen molecules combine to form ozone mole...
Questions


Mathematics, 23.07.2021 15:50

History, 23.07.2021 15:50

Mathematics, 23.07.2021 15:50

Computers and Technology, 23.07.2021 15:50

Mathematics, 23.07.2021 15:50


Mathematics, 23.07.2021 15:50

Mathematics, 23.07.2021 15:50







Mathematics, 23.07.2021 15:50


Mathematics, 23.07.2021 15:50


English, 23.07.2021 15:50

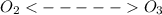 in presence of UV rays. Hence the rates of the forward and backward reactions remains the same in the atmosphere even after photo dissociation.
in presence of UV rays. Hence the rates of the forward and backward reactions remains the same in the atmosphere even after photo dissociation.

