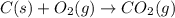The question: a new grill has a mass of 30.0 kg. you put 2.0 kg of charcoal in the grill. you burn all the charcoal and the grill has a mass of 30.0 kg. what is the mass in kg of the gases given off? (assume that the charcoal is pure carbon solid and that it burns completely in oxygen). the mass of carbon burnt = 2kg = 2000g moles of carbon burnt = 2000/12 => moles of co2 produced = 2000/12 => mass of co2 produced = 2000/12*44 = 2 333.33 g = 2.33 kg in the answer, where did the 44 come from?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 16:00
The overall chemical reaction for photosynthesis isshown below: 6co2 + 6h20 → c6h12o6 + 602what mass of glucose (c6h1206) can form from71.89 g co2? (molar mass of c6h1206 = 180.18g/mol; molar mass of co2 = 44.01 g/mol)71.89 g co2=g c6h1206
Answers: 1

Chemistry, 21.06.2019 17:00
In the analysis of hair and fiber samples, which does a compound comparison microscope allow for that a conventional compound microscope does not? a. simultaneous observation b. polarization c. fluorescence d. higher magnification
Answers: 2

Chemistry, 22.06.2019 02:10
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1
You know the right answer?
The question: a new grill has a mass of 30.0 kg. you put 2.0 kg of charcoal in the grill. you burn a...
Questions





Mathematics, 01.08.2021 23:30

Mathematics, 01.08.2021 23:30

Mathematics, 01.08.2021 23:30


Mathematics, 01.08.2021 23:30

Mathematics, 01.08.2021 23:30


Mathematics, 01.08.2021 23:30

Mathematics, 01.08.2021 23:30




English, 01.08.2021 23:30

Health, 01.08.2021 23:30


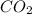 .
.