
Chemistry, 07.07.2019 22:00 brittanyfox411
During a water treatment program, 127 grams of calcium nitrate, ca(no3)2, is dissolved in water. the final volume of the solution is 2,300 milliliters. what is the molarity of the solution? refer to the periodic table to you answer. express your answer to two significant figures. the molarity of the solution is m.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:40
In the lab, ammonia was mixed with water to form ammonium hydroxide. what is/are the reactant(s)? o water and ammonia o ammonia o ammonium hydroxide need
Answers: 2


Chemistry, 23.06.2019 00:20
What type of context clue you understand the meaning of quandary?
Answers: 3

You know the right answer?
During a water treatment program, 127 grams of calcium nitrate, ca(no3)2, is dissolved in water. the...
Questions







Mathematics, 04.12.2020 16:30

Social Studies, 04.12.2020 16:30


Mathematics, 04.12.2020 16:30

Biology, 04.12.2020 16:30

Computers and Technology, 04.12.2020 16:30

Biology, 04.12.2020 16:30

English, 04.12.2020 16:30





Computers and Technology, 04.12.2020 16:30

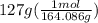
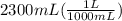
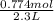 = 0.34 M
= 0.34 M

