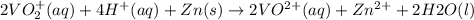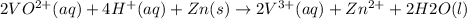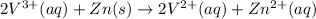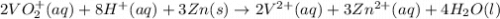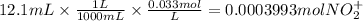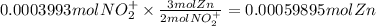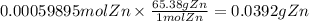Chemistry, 07.07.2019 19:30 tladitidimatso1783
The balanced redox reactions for the sequential reduction of vanadium are given below. reduction from +5 to +4: 2 vo2+(aq) + 4 h +(aq) + zn(s) → 2 vo2+(aq) + zn2+(aq) + 2 h2o(l) reduction from +4 to +3: 2 vo2+(aq) + zn(s) + 4 h +(aq) → 2 v3+(aq) + zn2+(aq) + 2 h2o(l) reduction from +3 to +2: 2 v3+(aq) + zn(s) → 2 v2+(aq) + zn2+(aq) if you had 12.1 ml of a 0.0033 m solution of vo2+(aq), how many grams of zn metal would be required to completely reduce the vanadium?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1

Chemistry, 23.06.2019 00:30
Maya wrote if you step to describe how carbon circulates between the atmosphere and living organisms
Answers: 1
You know the right answer?
The balanced redox reactions for the sequential reduction of vanadium are given below. reduction fro...
Questions

Mathematics, 03.03.2021 18:10




Mathematics, 03.03.2021 18:10


Mathematics, 03.03.2021 18:10


Mathematics, 03.03.2021 18:10

Social Studies, 03.03.2021 18:10

Chemistry, 03.03.2021 18:10

Social Studies, 03.03.2021 18:10

Mathematics, 03.03.2021 18:10


Mathematics, 03.03.2021 18:10



Health, 03.03.2021 18:10

Mathematics, 03.03.2021 18:10