Chemistry, 07.07.2019 18:30 cougar9754
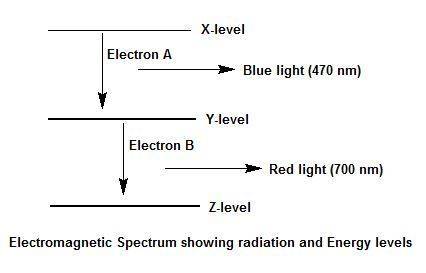
Electron a falls from energy level x to energy level y and releases blue light. electron b falls from energy level y to energy level z and releases red light. which transition, from x to y or from y to z, has a greater energy difference? explain your answer and how you arrived at it. use a diagram of the electromagnetic spectrum.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which type of reaction always has an element and a compound as reactants
Answers: 1

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3

Chemistry, 22.06.2019 12:00
What are the first two quantum numbers for the electrons located in subshell 4d? what are the first three quantum numbers for the electrons located in subshell 2s? how many electrons can be held in a sublevel l = 3? how many electrons can be held in the energy level n = 4? how many electrons in an atom can share the quantum numbers n = 4 and l = 3?
Answers: 1

Chemistry, 22.06.2019 23:00
What is the solubility-product constant of barium sulfate, baso4, if a saturated solution is 1.03 ´ 10-5 m?
Answers: 3
You know the right answer?
Electron a falls from energy level x to energy level y and releases blue light. electron b falls fro...
Questions


Mathematics, 23.03.2020 01:13

Mathematics, 23.03.2020 01:13

Mathematics, 23.03.2020 01:13





Mathematics, 23.03.2020 01:15


Mathematics, 23.03.2020 01:15


Mathematics, 23.03.2020 01:15



Mathematics, 23.03.2020 01:16

Mathematics, 23.03.2020 01:16

Mathematics, 23.03.2020 01:16

Mathematics, 23.03.2020 01:16

English, 23.03.2020 01:16

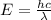
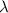 = Wavelength of particle
= Wavelength of particle