Chemistry, 07.07.2019 16:30 nancylagunas805
Determine the mass of 5.2 x 10 power of 21 molecules of propanol c3h7oh(l), on grams.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the energy released per kilogram of fuel used. given mev per reaction, calculate energy in joules per kilogram of reactants. consider 1 mole of tritium plus 1 mole of deuterium to be a mole of “reactions” (total molar mass = 5 grams).
Answers: 1

Chemistry, 21.06.2019 21:00
Consider the nuclear equation below. 239 > x + 4 he 94 2 what is x? 1.235 cm 96 2.243 u 92 3.235 u 92 4.243 cm 96
Answers: 2

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 08:00
Why is the bond angle in a water molecule less than the bond angle of methane? a. the central oxygen atom in water has two lone pairs of electrons, whereas the central carbon atom in methane has no lone pairs. b. the central hydrogen atom in water has one lone pair of electrons, whereas the central carbon atom in methane has two lone pairs. c. the central oxygen atom in water has four lone pairs of electrons, whereas the central carbon atom in methane has only one lone pair. d. the central oxygen atom exerts more repulsive force on surrounding atoms than the central carbon atom in methane does. reset next
Answers: 2
You know the right answer?
Determine the mass of 5.2 x 10 power of 21 molecules of propanol c3h7oh(l), on grams....
Questions

Mathematics, 20.04.2020 21:45

Mathematics, 20.04.2020 21:45

Arts, 20.04.2020 21:45

Mathematics, 20.04.2020 21:45


English, 20.04.2020 21:45

Biology, 20.04.2020 21:45


Mathematics, 20.04.2020 21:45

History, 20.04.2020 21:45

Social Studies, 20.04.2020 21:45


Chemistry, 20.04.2020 21:45

Mathematics, 20.04.2020 21:45

Mathematics, 20.04.2020 21:45

Mathematics, 20.04.2020 21:45

Mathematics, 20.04.2020 21:45



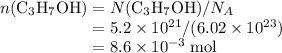
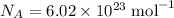 the Avogadro's constant that relates the number of particles to their number, in the unit moles
the Avogadro's constant that relates the number of particles to their number, in the unit moles 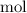 .
.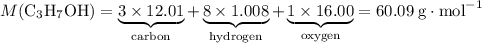
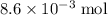 of propanol molecules would thus have a mass of
of propanol molecules would thus have a mass of